Water is Magic
Water is marvelous stuff and has many interesting properties. This is a good thing (for us) since these properties are necessary for life (as we know it) to exist.(This is an example of the anthropic principle--we are only able to observe water's interesting properties because we exist, and we are only able to exist because of water's interesting properties. So it is unavoidable that in our world--in any world where we could have evolved-- water must have such interesting properties.)Among water's most important properties is its high latent heat. This property creates much of Earth's most violent weather and drives the thermodynamics of climate.
What is "Latent Heat"?
To understand latent heat you first have to understand the idea of states of matter and phase changes.Chemical substances can exist in several "states". The common ones that we encounter in everyday life are the solid state, the liquid state, and the gaseous state. When matter changes from one state to another we call it a "phase change". So liquid water can undergo a phase change to become the gas water vapor, and it can reverse that transition and condense from a gas into a liquid. It can undergo a different phase change from a liquid to become solid ice, and the reverse to melt from ice into liquid. See the diagram below.
 |
| The names of the various common states of matter and of the phase transitions between them |
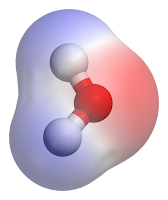
To evaporate one kilogram of water by boiling it, changing it from liquid to gas at 100 degrees C, takes 2,260 kilojoules. That is about two and a half times as much energy as is needed to vaporize a kilogram of ethyl alcohol.Water molecules are highly polar molecules. This means they have uneven distribution of their electrons, with more electrons bunching around the oxygen atom and thus creating lower electron density around the hydrogen atoms. So the molecule is more negative on one side (where the electrons are concentrated) and more positive on the other side. It is like a little magnet, a dipole. As you are no doubt aware, "opposites attract". So the negative side, or middle, of a water molecule will tend to attract the positive sides, or ends, of other water molecules. This is an example of "hydrogen bonding". Hydrogen bonding is extremely important in the machinery of life. This attraction tends to hold the water molecules in liquid water together. To evaporate water--to make some of those molecules break away from the liquid mass and fly off as a gas--takes a lot of energy.
illustrative separation of
charges on water molecule:
negative red, positive blue
Latent Heat in the Kitchen
 |
| Pot of boiling water |
The truly amazing thing about the latent heat of evaporation of water is that when the phase change is reversed, when water vapor condenses into liquid water, the same amount of heat is released. This isn't as obvious to us as the amount of heat consumed in boiling, but you may have experienced it if you have gotten your hand in the stream of steam escaping from a teakettle.
This is the reasons that burns caused by steam can be so severe. Besides the heat of the steam, some of the steam will condense on the skin, releasing its latent heat of condensation. This is equal to the latent heat of vaporization of the same amount of water. You wouldn't want to put your hand in the flame needed to vaporize even a small amount of water. But when that small amount of water condenses out of steam on your skin it releases just that amount of heat. This is why a burn from steam can be more severe than a burn by boiling water itself, if the quantity of steam is significant.
How Latent Heat Drives Storms
So when water evaporates it takes up heat (cooling the local environment). As water vapor it carries that heat around as latent heat. Then when that vapor condenses it releases that latent heat, heating up the local environment, usually the air.This is what drives some types of storms, including thunderstorms, tornadoes, hurricanes and typhoons. Such storms are driven by "heat engines" based on water vapor. The key to such systems is rising warm air containing water vapor. As it rises it expands (because the atmospheric pressure is lower the higher you go) and as it expands it cools (the same amount of heat is spread through a larger volume--adiabatic cooling).
At some point the parcel of moist air has cooled enough that it cannot hold all the water vapor it contains. (The amount of water vapor air can hold is strongly dependent on its temperature.) So some of the water vapor condenses out as water droplets--clouds, rain or snow. As that water vapor condenses to liquid it releases heat (the latent heat of condensation or latent heat of fusion), warming the parcel of air. Because of this warming, the moist parcel of air will be warmer and more buoyant than neighboring air, so it will continue to rise.
As it rises and expands more condensation will occur, continuing the process. (This gives rise to towering "thunderhead" cloud formations.) Essentially this creates a strong updraft as water condenses out of the rising air. This updraft causes locally lower air pressure below it and sucks in surrounding air to fill the gap, creating surface wind--the storm as we experience it. (There may also be downdrafts associated with falling precipitation.)
Without the heat released by the condensation of water vapor these systems couldn't grow to their towering size.
 |
| Tropical cyclone driven by energy released by condensation of moisture |
Latent Heat of Evaporation and Climate Change
There are several processes where the latent heat of water becomes important in trying to understand climate change associated with global warming.Tropical Cyclones
Increases in sea surface temperatures could affect the formation and behavior of hurricanes. As noted above, warm ocean waters put a lot of moisture into the air (the air can hold more moisture at higher temperature), and it is water vapor in the air that makes the hurricane heat engine work once one gets started. Wider areas of warm ocean waters could mean tropical cyclones will form in places they didn't form before.
There is a lot of scientific discussion about this because warming causes other simultaneous changes. For instance, hurricanes can't form if the local winds are too high--only where there are just light breezes. Will warming change the distribution of winds over warm areas of the seas?
There is some evidence that Atlantic hurricane numbers have been rising (previous post) but this is still in dispute.
If seas are warmer they might also contribute to the strength of tropical cyclones that do form. This previous post discusses research that suggests increased destructiveness of hurricanes associated with warmer seas. This question is still not settled though.
Cooling By Irrigation
Because of the latent heat of water, more evaporation means more cooling in some places, and more rain means more warming in other places. A recent article in the Journal of Geophysical Research (pdf here, New York Times Green blog post about it here) says irrigation may be causing cooling in some regions, locally masking the effects of global warming.
The model runs reported in this paper suggest that parts of norther India may have experienced several degrees of cooling due to all the heat absorbed by irrigation water applied to crops in the later part of the 20th century. Weather patterns may even have been affected enough to reduce the amount of rain in the Bay of Bengal branch of the Southwest Monsoon. (Other researchers got somewhat different or even contradictory results with different models.)
This is a bit scary because if groundwater depletion leads to reduction in irrigation in the future, the reduction of cooling effect could have both local an regional climate effects, including sharply higher temperatures and changes in rainfall amounts and distribution.
Evaporative Thermoregulation
Evaporation is used to cool the bodies of many animals. Sweat evaporating from the skin makes it possible for us to deal with hot weather. On a hot day in dry weather a person can lose more than a liter of water by evaporation of sweat (even several liters if it is really hot or you are exercising). Think of the amount of heat it would take to boil away a liter of water.Other Uses of Latent Heat
There are many other uses of the latent heat of water for cooling, for example:- Evaporative coolers, a kind of air conditioning.
- Some cooling towers at power plants
and other industrial facilities use evaporative cooling. Steam turbines require a condenser to cool the steam after it leaves the turbine so that it condenses into water and can be pumped back through the cycle. Such condensers often use evaporative cooling by spraying or dripping water over coils carrying hot water from the system. At big power plants these may be enclosed in characteristic hyperboloid chimney-like structures to provide draft to move the moisture-laden air out of the cooling unit. Other systems use fans. [Here is a video of the inside of a cooling tower showing water being sprayed over cooling circuits.]
Cooling towers at Didcot Power Station,
Understand latent heat and many phenomena will be less mysterious to you.
The diagram of phase changes is a public domain image from Wikimedia Commons. Rights information here.
The illustration of charges on a water molecule has been placed in the public domain by its author, from Wikimedia Commons. Rights information here.
The picture of the pot of boiling water is from Wikimedia Commons, with the permission of the copyright holder under the terms of the GNU Free Documentation License.
Diagram of tropical cyclone by Jannev, placed in the public domain at Wikimedia Commons.
Picture of Didcot Power Station by Dave Price from Wikimedia Commons, used under a Creative Commons Attribution Share-alike license 2.0
David Wheat's Science In Action site has articles about science and math in the real world, weird science, science news, unexpected connections, and other cool science stuff. There is an index of the articles by topic here.

2 comments:
An excellent precis of latent heat, Please don't let the bad science of global warming cloud your judgement or purety of scientific thought. Global cooling and another ice age is what we need to worry about with 8 billion mouths to feed and water(ice)Any discussion on hydrogen bonding should include the density of ice anomaly
Excellent article. Exactly the level i was looking for to kog my schoolboy memories of latent heat and evaporation.
Post a Comment